Integrating HAZOP into Risk Management Systems
Hazard and Operability Studies (HAZOP) are systematic, multidisciplinary analyses of process units or operations that identify how deviations from design intent can create hazards. A HAZOP is typically conducted by a cross‐functional team (engineers, operators, safety specialists, etc.) who use guidewords (e.g. “more/less”, “no”, “reverse”) on each process node (equipment, line, or procedure) to uncover potential failures. For example, one infographic illustrates HAZOP as a stepwise process: form the team → apply guidewords at each node → identify deviations → evaluate their causes and consequences → develop recommendations.
In practice, HAZOP requires up‐to‐date process documentation (PFDs/P&IDs), an experienced facilitator, and structured reporting. Its theoretical foundation is rooted in process hazard analysis (PHA) methodologies, and it is widely regarded as an international best practice for identifying hazards and operability problems in complex processes. In fact, HAZOP has been called the “foundation of process safety and risk management programs” in the chemical and petroleum industries.
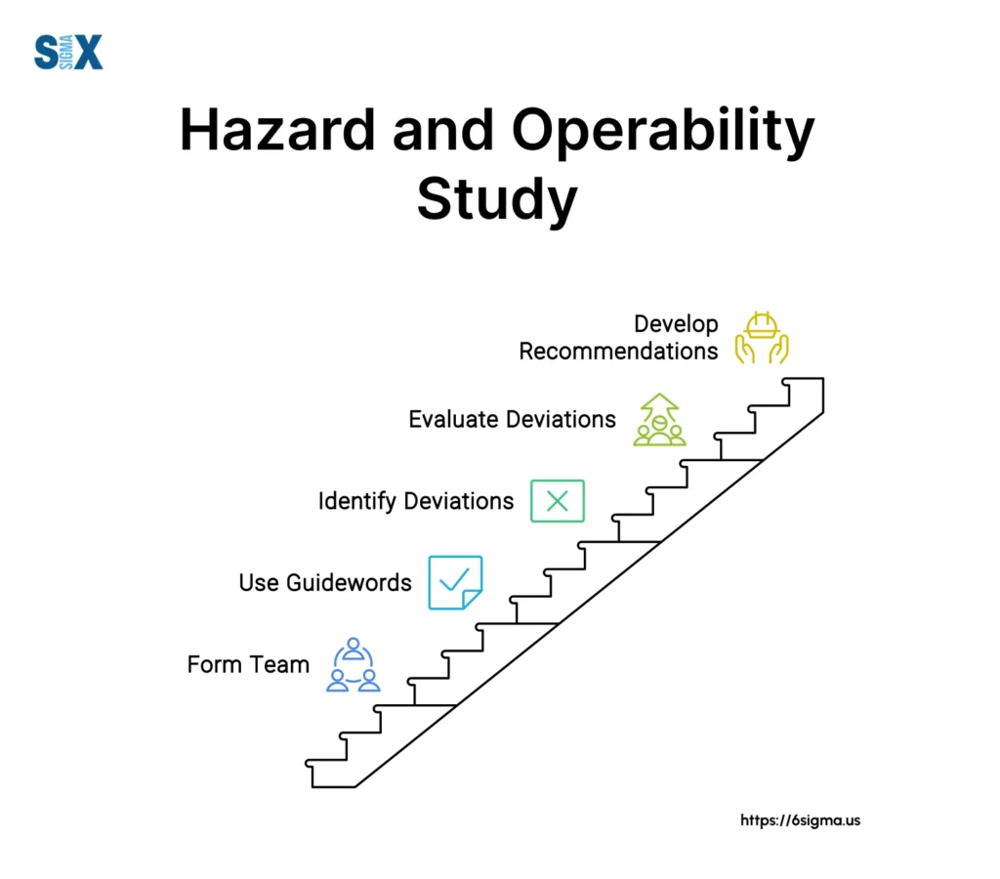
Figure 1: A HAZOP team works methodically through process “nodes” using guidewords to identify deviations, analyze causes, and develop risk mitigation recommendations
By proactively detecting deviations, HAZOP helps reduce safety, health, environmental and reliability risks. Studies have shown HAZOPs surface hidden failure modes and guide robust safeguards. For example, in a crude oil production plant HAZOP identified 71 risk scenarios and generated 47 mitigation actions (e.g. new alarms, sensors, relief devices). Similarly, in pharmaceutical tablet manufacturing a HAZOP revealed critical causes of quality issues (dose variation, dissolution failures), leading to improved controls and adherence to ICH Q9 Quality Risk Management guidance.
These cases illustrate HAZOP’s dual benefit: improving process safety while bolstering overall risk awareness, decision-making, and regulatory compliance.
Click HERE for Process Safety (HAZOP Study, LOPA, QRA, HIRA, SIS), Quality Management, Engineering, ISO Management Systems, Project Management, Lean Six Sigma & Process Improvement Self-paced Training Courses
Alignment with Risk Management Standards
A key advantage of HAZOP is its compatibility with formal risk management frameworks. For instance, ISO 31000:2009 defines risk management as an integral, organization-wide process that supports decision-making. ISO 31000 breaks risk management into stages (Figure 1), with Risk Assessment (identification, analysis, evaluation) at its core. A HAZOP naturally maps onto this framework: it is the risk identification and qualitative analysis step.
Preparation of a HAZOP (scope definition, team selection, criteria, documentation) corresponds to “establish context” and “risk analysis” in ISO 31000. The output of HAZOP – hazards, causes, consequences, and recommended safeguards – feeds directly into risk evaluation and risk treatment plans. Because ISO 31000 emphasizes integrating risk management into all processes, HAZOP findings should feed into the organization’s risk register, safety management system, and management-of-change processes
Likewise, many industry regulations call out HAZOP (or equivalent PHA methods) explicitly. In the United States, OSHA’s Process Safety Management (PSM) standard (29 CFR 1910.119) requires covered facilities to perform a Process Hazard Analysis that “shall be appropriate to the complexity of the process”. Acceptable PHA methodologies include HAZOP, “What‑If”, FMEA, FTA and equivalents. Thus OSHA PSM essentially mandates regular HAZOP (or similar studies) for hazardous chemical processes, with multidisciplinary teams and follow-up on recommendations.
Compliance with API RP 754 (“Process Safety Performance Indicators”) also benefits from a strong HAZOP program: HAZOP insights contribute leading indicators (e.g. actions initiated, safety barriers installed) and inform causal analysis of Tier 1/2 safety events. In healthcare pharmaceuticals, the ICH Q9 guideline (Quality Risk Management) similarly recognizes structured risk analysis like HAZOP as part of a robust Quality Management System. In short, HAZOP is fully compatible with – and often required by – risk management standards across oil & gas, pharmaceuticals, and manufacturing.

Figure 2: A multidisciplinary HAZOP team reviews process drawings and brainstorms deviations. Effective HAZOP integration relies on cross‐functional participation, clear documentation, and timely follow-up to turn findings into safety improvements
Click HERE for Process Safety (HAZOP Study, LOPA, QRA, HIRA, SIS), Quality Management, Engineering, ISO Management Systems, Project Management, Lean Six Sigma & Process Improvement Self-paced Training Courses
Figure 2 (above) shows a typical HAZOP team session. Best practice is to involve process designers, operations personnel, and maintenance engineers so that controls can be both technically sound and operationally practical. After the HAZOP, the findings should be communicated (via reports or dashboards) to all stakeholders – including management, operations, maintenance, and safety – so that everyone sees the link between identified hazards and necessary controls. HAZOP results should feed into formal SMS/PSM elements such as:
- Hazard registers and risk matrices: input identified scenarios into a living risk register or bow-tie analysis for corporate oversight.
- Management of Change (MOC): when equipment/process changes are proposed, update or re-run relevant HAZOP sections.
- Training and Procedures: update operating procedures and train staff on new safeguards identified by HAZOP.
- Incident investigation and learning: use HAZOP logic to analyze incidents and verify whether HAZOP had anticipated the scenario or missed it.
Integrating HAZOP with KPIs and audits completes the loop. For example, organizations can track metrics such as “percent HAZOP action items closed” or “time to close HAZOP recommendations” as leading performance indicators (aligned with API 754 Tier 0 metrics). Periodic safety audits (or process safety reviews) should verify that HAZOP findings have been addressed and that no new deviations have arisen since the study. In short, HAZOP must be part of the PDCA (Plan-Do-Check-Act) cycle of the safety management system: plan (HAZOP study) → do (implement actions) → check (audit results) → act (update processes, re-assess).
Click HERE for Process Safety (HAZOP Study, LOPA, QRA, HIRA, SIS), Quality Management, Engineering, ISO Management Systems, Project Management, Lean Six Sigma & Process Improvement Self-paced Training Courses
Challenges and Limitations
Despite its strengths, integrating HAZOP poses challenges. A primary concern is resource intensity. A thorough HAZOP (covering an entire plant) can take weeks of team effort. Smaller operations may struggle to dedicate skilled engineers and time away from production. Without experienced facilitators and complete documentation, HAZOP sessions can become unfocused or superficial. In practice, “teams can lose scenarios, participants can become complacent, [and] the process can be complex and prolonged”. To mitigate this, many practitioners segment large facilities into smaller HAZOP nodes or use software tools to manage information flow.
Another challenge is integration into existing systems. If HAZOP is done in isolation, its recommendations may languish unaddressed. Organizations must ensure cross-departmental ownership of findings. There may also be overlap or tension between HAZOP and other risk analyses (e.g. FMEA or BowTie) if not well coordinated. Integrating HAZOP into broader risk frameworks requires clear interfaces: for example, linking HAZOP hazard logs to the corporate risk register or bow-tie diagrams. Also, metrics from one system (like API 754 incident counts) may not directly reflect HAZOP outcomes, so care is needed to draw connections between them.
Human and organizational factors can limit effectiveness. As one study notes, HAZOP often omits detailed human factors analysis. The oil-production HAZOP authors pointed out that risks from “human negligence” were not explicitly captured, and recommended supplementing classic HAZOP with procedural reviews or human-HAZOP steps. Team skill level matters too; less experienced teams may miss complex causes or misjudge safeguard effectiveness. Finally, there is always the risk of HAZOP becoming a “paper exercise” if management does not act on it. Sustaining impact means establishing accountability for HAZOP action items as part of normal operations.
Industry Case Studies
- Oil & Gas: A recent applied study in a crude-oil production unit (perforation, separation, pumping) divided the facility into nodes and ran a HAZOP workshop. The team identified 80 causes and 71 hazardous scenarios, leading to 60 new safeguards/barriers and 47 action recommendations. Key findings included latent design flaws (e.g. inadequate relief valves) and equipment faults. Mitigations ranged from installing high-temperature alarms and level transmitters to relocating vessels away from occupied areas. The study concluded that HAZOP “provides essential knowledge for company leaders and decision-makers” and remains “an excellent risk analysis tool” for oil facilities, despite requiring experienced teams and supplementary analysis for human error.
- Pharmaceuticals: In a tablet manufacturing line, a formal HAZOP was used to address quality and safety risks. The HAZOP team examined coating and compression steps, identifying non-obvious causes of dose and dissolution variability (e.g. inconsistent spray rates, feeder faults). The result was a series of quality risk controls – from in-line weight/dissolution monitoring to updated SOPs – that brought process parameters into specification. This case demonstrated HAZOP as a Quality Risk Management (QRM) tool: by aligning with ICH Q9 and GMP, it not only fixed critical quality issues but also “promoted operational discipline, regulatory preparedness, and a continuous improvement culture”. Auditors and regulators valued the documented, systematic approach.
- Manufacturing (Automotive): An automobile components plant implemented HAZOP to tackle chronic production snags. In one example, a Gurgaon-based parts factory used HAZOP to analyze recurring machine breakdowns. The study revealed several “critical failure points that had previously gone unnoticed.” Armed with these insights, the firm adopted targeted preventive maintenance schedules and upgraded key equipment, which significantly reduced downtime and boosted productivity. In another case, a manufacturer built HAZOP into its new-product development. Early hazard analysis of design and process options guided material selections and production methods. This upfront risk assessment “enhanced product safety” and reduced costly redesigns later in development. These success stories underscore that HAZOP can drive both safety and efficiency gains when embedded across operations.
Click HERE for Process Safety (HAZOP Study, LOPA, QRA, HIRA, SIS), Quality Management, Engineering, ISO Management Systems, Project Management, Lean Six Sigma & Process Improvement Self-paced Training Courses
Best Practices and Recommendations
To harness HAZOP’s full potential within risk management:
- Integrate with ISO 31000/PSM processes: Treat HAZOP as the standard PHA technique. Align it with the organization’s risk management cycle – from context-setting to continuous review. Include HAZOP inputs in risk registers, management reviews, and improvement planning.
- Engage multidisciplinary teams: Assemble cross-functional HAZOP teams (engineering, operations, maintenance, safety, quality) and experienced facilitators. Ensure at least one process expert per discipline. This diversity is critical for thorough coverage of failure modes and practical solutions.
- Maintain up-to-date documentation: Before each HAZOP, verify that PFDs/P&IDs, process data (inventory, limits, material specs) and procedures are current. Accurate inputs (mass balances, operating manuals) make the difference between credible analysis and missed hazards.
- Prioritize and track actions: Not all HAZOP findings carry equal risk. Use risk ranking (e.g. risk matrix or semi-quantitative scoring) to prioritize follow-up. Assign each recommendation to a responsible owner with a deadline. Integrate these tasks into MOC and maintenance planning. Regularly review action completion in management-of-change and safety meetings.
- Use software tools and visual outputs: Digitize HAZOP records to allow filtering and reporting of hazards. Dashboards can link HAZOP items to KPIs. Present HAZOP results in clear formats (e.g. bow-tie diagrams, prioritized lists, heat maps) to communicate with executives and shop-floor staff.
- Train and audit: Provide HAZOP training (including refreshers) for engineers and operators. Encourage certification (e.g. IChemE HAZOP courses) for facilitators. Conduct periodic audits to verify that implemented safeguards still function and that no new hazards have emerged since the last HAZOP.
- Link to performance indicators: Define leading metrics (e.g. fraction of high-risk HAZOP recommendations closed on time) and lagging metrics (e.g. reduction in Tier 1/2 events) to measure HAZOP effectiveness in the API 754 framework. Show how HAZOP outcomes improve process safety performance over time.
- Scale to organizational context: Tailor HAZOP scope and frequency to business needs. For very complex or novel systems, consider layered studies (initial screening HAZOPs followed by detailed HAZOPs). Incorporate lessons learned – if a similar unit elsewhere in the company had incidents, ensure those scenarios are checked in the new HAZOP.
Conclusion
In high-risk industries, HAZOP remains a cornerstone of risk management. By systematically identifying how equipment or process deviations can create hazards, HAZOP provides the raw hazard data that feeds ISO 31000-style risk assessments, OSHA‐style PSM programs, and API 754 safety metrics. When HAZOP is fully integrated – with clear procedures for documentation, action-tracking, and follow-up – it transforms from a stand-alone study into an embedded element of the organization’s safety culture. This integrated approach not only enhances regulatory compliance (by satisfying PSM/PQI requirements) but also drives operational excellence: it reveals hidden risks before they cause accidents, informs smarter design and maintenance, and supports continuous improvement loops.
In practice, the benefits of integrated HAZOP include improved hazard visibility, stronger safety culture, and prevention of both safety and quality incidents. Challenges (resource needs, complexity, human factors) are mitigated by careful planning, segmentation of studies, and management commitment to act on findings. Our recommended best practices – from forming well‐rounded teams to linking findings with management-of-change and performance indicators – help ensure that HAZOP does not sit on a shelf but actively drives risk reduction across oil & gas, pharmaceutical, and manufacturing operations. As illustrated by industry cases, organizations that embed HAZOP in their risk management system reap tangible safety and efficiency gains, ultimately making processes safer, more reliable, and more resilient.
Click HERE for Process Safety (HAZOP Study, LOPA, QRA, HIRA, SIS), Quality Management, Engineering, ISO Management Systems, Project Management, Lean Six Sigma & Process Improvement Self-paced Training Courses
- Lean Six Sigma Yellow Belt Certification Course
- Lean Six Sigma Green Belt Certification
- Lean Six Sigma Black Belt Certification
- Lean Manufacturing Certification Course
- Six Sigma for Manufacturing & Operational Efficiency
- Statistical Process Control (SPC) Training Course
- Measurement Systems Analysis (MSA) Training Course
- Strategic Supply Chain Management Training Course
- Operational Risk Management Training Course
- Quality Management Training Course
- Health, Safety and Environment (HSE) Training Course
- Industrial Process Automation Training Course
- Advanced Digital Manufacturing & Product Design Technologies Training Courses
- Advanced Digital Manufacturing & Product Design Technologies Training Courses
- Integrated Management Systems (IMS) Implementation Masterclass
- Integrated ISO Management Systems (IMS) Audit – Best Practices
- ISO 19011: Auditing Management Systems – Techniques & Best Practices
- ISO 9001 Quality Management Systems Lead Auditor
- ISO 14001 Environmental Management Systems Lead Auditor
- ISO 45001 Occupational Health & Safety Lead Auditor
- ISO 50001 Energy Management Systems Lead Auditor
- Food Safety Management Systems (FSMS) Implementation
- ISO 22000 Food Safety Management Systems Lead Auditor
- FSSC 22000 Lead Auditor (Food Safety)
- ISO/IEC 17025 Laboratory Management Systems (LMS) Certification
- Laboratory Management Systems (LMS) Essentials
- Safety Instrumented System
- Hazards and Operability Study (HAZOP)
- Layer of Protection Analysis (LOPA)
- Quantitative Risk Assessment (QRA)
- Process Safety Management (PSM)
- Industrial Process Safety